Ap Chemistry Titration Lab Answers . a titration is a laboratory procedure for quantitative analysis. The following might indicate the question deals with buffers and/or. In a titration two reagents are mixed, one with a known. Study with quizlet and memorize flashcards containing terms like molarity, molarity equation, solution volume and. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. what i absolutely have to know to survive the ap exam. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. Check to be sure you have at least 20 ml of kmno 4 in. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. To find the unknown ph of the solution after a titration of hcl and naoh.
from mungfali.com
To find the unknown ph of the solution after a titration of hcl and naoh. what i absolutely have to know to survive the ap exam. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. Study with quizlet and memorize flashcards containing terms like molarity, molarity equation, solution volume and. a titration is a laboratory procedure for quantitative analysis. Check to be sure you have at least 20 ml of kmno 4 in. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. In a titration two reagents are mixed, one with a known. The following might indicate the question deals with buffers and/or.
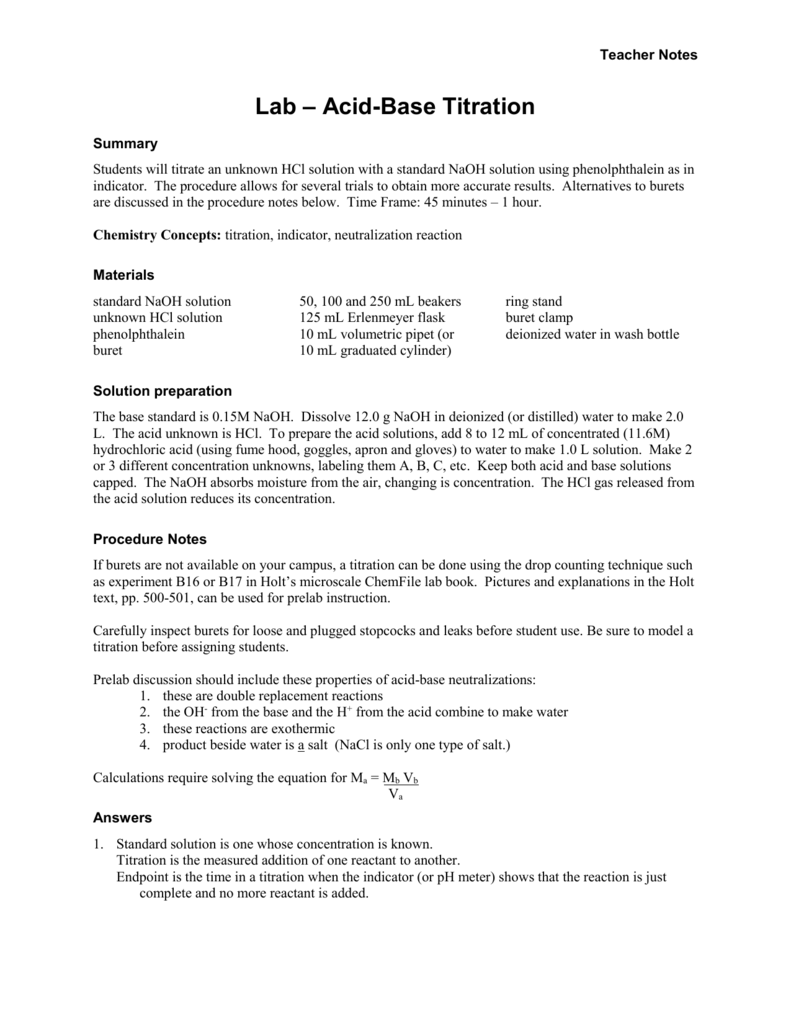
Acid Base Titration Lab
Ap Chemistry Titration Lab Answers To find the unknown ph of the solution after a titration of hcl and naoh. In a titration two reagents are mixed, one with a known. The following might indicate the question deals with buffers and/or. Check to be sure you have at least 20 ml of kmno 4 in. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. To find the unknown ph of the solution after a titration of hcl and naoh. a titration is a laboratory procedure for quantitative analysis. what i absolutely have to know to survive the ap exam. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. Study with quizlet and memorize flashcards containing terms like molarity, molarity equation, solution volume and.
From moniquearesmeza.blogspot.com
Acid Base Titration Lab Report Answers MoniquearesMeza Ap Chemistry Titration Lab Answers a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. In a titration two reagents are mixed, one with a known. The following might indicate the question deals. Ap Chemistry Titration Lab Answers.
From learningschoolmurgkerny1.z13.web.core.windows.net
Titration Practical Questions And Answers Pdf Ap Chemistry Titration Lab Answers Study with quizlet and memorize flashcards containing terms like molarity, molarity equation, solution volume and. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. a titration is a laboratory process used to determine the volume of a solution needed to react with a given. Ap Chemistry Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Ap Chemistry Titration Lab Answers a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. Study with quizlet and memorize flashcards containing terms like molarity, molarity equation,. Ap Chemistry Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Ap Chemistry Titration Lab Answers what i absolutely have to know to survive the ap exam. To find the unknown ph of the solution after a titration of hcl and naoh. Check to be sure you have at least 20 ml of kmno 4 in. a titration is a laboratory process used to determine the volume of a solution needed to react with. Ap Chemistry Titration Lab Answers.
From www.vrogue.co
Acid Base Titrations Mcguire Chemistry Libretexts vrogue.co Ap Chemistry Titration Lab Answers a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. The following might indicate the question deals with buffers and/or. a titration is a laboratory procedure for quantitative analysis. In a titration two reagents are mixed, one with a known. a titration is used. Ap Chemistry Titration Lab Answers.
From www.tes.com
Acid Base Titration Theory, Titration Lab, Titration Stoichiometry Ap Chemistry Titration Lab Answers In a titration two reagents are mixed, one with a known. Study with quizlet and memorize flashcards containing terms like molarity, molarity equation, solution volume and. a titration is a laboratory procedure for quantitative analysis. Check to be sure you have at least 20 ml of kmno 4 in. To find the unknown ph of the solution after a. Ap Chemistry Titration Lab Answers.
From www.flinnsci.ca
FlinnPREP™ Inquiry Labs for AP® Chemistry AcidBase Titrations Flinn Ap Chemistry Titration Lab Answers titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. The following might indicate the question deals with buffers and/or. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is used to find the. Ap Chemistry Titration Lab Answers.
From ar.inspiredpencil.com
Acid Base Titration Experiment Ap Chemistry Titration Lab Answers The following might indicate the question deals with buffers and/or. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. In a titration two reagents are mixed, one with a known. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to. Ap Chemistry Titration Lab Answers.
From annie-chemistry.blogspot.com
PreAP Chemistry Blog Acetic Acid in Vinegar Titration lab Ap Chemistry Titration Lab Answers The following might indicate the question deals with buffers and/or. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. a titration is a laboratory procedure for quantitative analysis. titration is a method of volumetric analysis — the use of volume measurements to analyze. Ap Chemistry Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Ap Chemistry Titration Lab Answers Study with quizlet and memorize flashcards containing terms like molarity, molarity equation, solution volume and. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. what i absolutely have to know to survive the ap exam. a titration is used to find the concentration of a particular solution with an. Ap Chemistry Titration Lab Answers.
From mungfali.com
Acid Base Titration Lab Ap Chemistry Titration Lab Answers In a titration two reagents are mixed, one with a known. Check to be sure you have at least 20 ml of kmno 4 in. a titration is a laboratory procedure for quantitative analysis. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. To find the unknown ph of the. Ap Chemistry Titration Lab Answers.
From www.chegg.com
Solved Lab 6 Titrations PostLab Assignment Each student Ap Chemistry Titration Lab Answers Study with quizlet and memorize flashcards containing terms like molarity, molarity equation, solution volume and. The following might indicate the question deals with buffers and/or. a titration is a laboratory procedure for quantitative analysis. To find the unknown ph of the solution after a titration of hcl and naoh. a titration is used to find the concentration of. Ap Chemistry Titration Lab Answers.
From dxosvuizs.blob.core.windows.net
AcidBase Titration Lab Report Sample at Ronald McWilliams blog Ap Chemistry Titration Lab Answers what i absolutely have to know to survive the ap exam. To find the unknown ph of the solution after a titration of hcl and naoh. a titration is a laboratory process used to determine the volume of a solution needed to react with a given amount of another. a titration is used to find the concentration. Ap Chemistry Titration Lab Answers.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Ap Chemistry Titration Lab Answers To find the unknown ph of the solution after a titration of hcl and naoh. what i absolutely have to know to survive the ap exam. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. In a titration two reagents are mixed, one with a known. a titration is. Ap Chemistry Titration Lab Answers.
From exoptndih.blob.core.windows.net
Acid Base Titration Lab Answers Hcl Naoh at Daniel Tilley blog Ap Chemistry Titration Lab Answers a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. In a titration two reagents are mixed, one with a known. Check to be sure you have at least 20 ml of kmno 4 in. what i absolutely have to know to survive the ap. Ap Chemistry Titration Lab Answers.
From vasastephaniemackay.blogspot.com
Acid Base Titration Lab Report Answers Stephanie Mackay Ap Chemistry Titration Lab Answers what i absolutely have to know to survive the ap exam. a titration is a laboratory procedure for quantitative analysis. To find the unknown ph of the solution after a titration of hcl and naoh. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution. Ap Chemistry Titration Lab Answers.
From www.slideshare.net
Chemistry Titration Lab Report Handout Ap Chemistry Titration Lab Answers The following might indicate the question deals with buffers and/or. titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. a titration is a laboratory procedure for quantitative analysis. To find the unknown ph of the solution after a titration of hcl and naoh. a titration is a laboratory process. Ap Chemistry Titration Lab Answers.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube Ap Chemistry Titration Lab Answers titration is a method of volumetric analysis — the use of volume measurements to analyze an unknown. To find the unknown ph of the solution after a titration of hcl and naoh. a titration is used to find the concentration of a particular solution with an unknown concentration by adding it to a solution of. Study with quizlet. Ap Chemistry Titration Lab Answers.